Performance Meter
0%
QUESTION ID:1
QUESTION ID:2
QUESTION ID:3
QUESTION ID:4
QUESTION ID:5
QUESTION ID:6
QUESTION ID:7
QUESTION ID:8
QUESTION ID:9
QUESTION ID:10
QUESTION ID:11
QUESTION ID:12
The total number of chair conformations possible for 1,2-dimethylcyclohexane is _____.
QUESTION ID:13
QUESTION ID:14
QUESTION ID:15
QUESTION ID:16
QUESTION ID:17
QUESTION ID:18
QUESTION ID:19
QUESTION ID:20
QUESTION ID:21
The major product formed in the following reaction is (ignore product stereochemistry)
QUESTION ID:22
QUESTION ID:23
The major product formed in the following reaction is
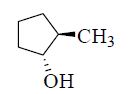
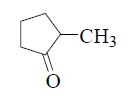
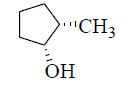
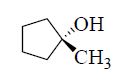
QUESTION ID:24
QUESTION ID:25
QUESTION ID:26
QUESTION ID:27
Hydrolysis of a peptide involves cleavage of the bond between the atoms
QUESTION ID:28
QUESTION ID:29
QUESTION ID:30
QUESTION ID:31
QUESTION ID:32
QUESTION ID:33
QUESTION ID:34
QUESTION ID:35
QUESTION ID:36
QUESTION ID:37
QUESTION ID:38
QUESTION ID:39
QUESTION ID:40
QUESTION ID:41
QUESTION ID:42
QUESTION ID:43
QUESTION ID:44
QUESTION ID:45
QUESTION ID:46
QUESTION ID:47
QUESTION ID:48
QUESTION ID:49
QUESTION ID:50
QUESTION ID:51
QUESTION ID:52
QUESTION ID:53
QUESTION ID:54
QUESTION ID:55
QUESTION ID:56
QUESTION ID:57
QUESTION ID:58
QUESTION ID:59
QUESTION ID:60
QUESTION ID:61
QUESTION ID:62
QUESTION ID:63
QUESTION ID:64
| Gene/enzyme | Commercial use |
| P. Bt gene | i. Golden rice |
Q. β-carotene biosynthetic genes | ii. insect resistance |
| R. ACC deaminase | iii. herbicide resistance |
| S. EPSP synthase | iv. fruit ripening |
QUESTION ID:65
QUESTION ID:66
QUESTION ID:67
QUESTION ID:68
QUESTION ID:69
QUESTION ID:70
QUESTION ID:71
QUESTION ID:72
QUESTION ID:73
QUESTION ID:74
The net yield of NADH in the Embden-Meyerhof pathway in E. coli is________.
QUESTION ID:75
QUESTION ID:76
QUESTION ID:77
QUESTION ID:78
QUESTION ID:79
QUESTION ID:80
QUESTION ID:81
QUESTION ID:82
QUESTION ID:83
QUESTION ID:84
| Group I | Group II |
| (P) Cell membrane | (I) Nutrient transport |
| (Q) Purple membrane | (II) Photosynthesis |
| (R) Cisternae | (III) Active transport |
| (S) Outer membrane | (IV) Protein glycosylation |
| (V) Light-driven proton transport |
QUESTION ID:85
| Group I | Group II |
| (P) Nalidixic acid | (I) RNA polymerase |
| (Q) Tetracycline | (II) DNA gyrase |
| (R) Erythromycin | (III) DNA polymerase |
| (S) Rifampin | (IV) 50S ribosomal subunit |
| (V) Aminoacyl tRNA |
QUESTION ID:86
QUESTION ID:87
QUESTION ID:88
QUESTION ID:89
QUESTION ID:90
QUESTION ID:91
QUESTION ID:92
QUESTION ID:93
QUESTION ID:94
QUESTION ID:95
QUESTION ID:96
QUESTION ID:97
QUESTION ID:98
QUESTION ID:99
QUESTION ID:100
QUESTION ID:101
QUESTION ID:102
QUESTION ID:103
QUESTION ID:104
QUESTION ID:105
Given below is the list of animals and their respective characteristics.
| Animals | Characteristics |
| I. Sea anemone | i. Three pairs of jointed legs |
| II. Bluefly | ii. Diploblastic acoelomate |
| III. Starfish | iii. Collar cells |
| IV. Sponge | iv. Tube feet |
Which ONE of the following represents the correct match?
QUESTION ID:106
QUESTION ID:107
QUESTION ID:108
QUESTION ID:109
QUESTION ID:110
QUESTION ID:111
QUESTION ID:112
QUESTION ID:113
QUESTION ID:114
QUESTION ID:115
QUESTION ID:116
| Column I | Column II |
| P. Clostridium botulinum | 1. Fish |
| Q. Salmonella spp. | 2. Cooked starch foods |
| R. Vibrio parahaemolyticus | 3. Meat, egg and poultry |
| S. Bacillus cereus | 4. Canned foods |
QUESTION ID:117
QUESTION ID:118
QUESTION ID:119
QUESTION ID:120
QUESTION ID:121
QUESTION ID:122
| Column I | Column II |
| P. Amylase | 1. Conversion of sucrose to glucose and fructose |
| Q. Invertase | 2. Softening of dough |
| R. Phosphatase | 3. Effectiveness of pasteurization |
| S. Protease | 4. Conversion of starch to maltose |
QUESTION ID:123
| Column I | Column II |
| P. Enrichment | 1. Overcome the deficiency of nutrients by mixing of two plant sources |
| Q. Fortification | 2. Overcome the deficiency of nutrients from a synthetic source |
| R. Supplementation | 3. Restoration of nutrients which are lost during processing |
| S. Complementation | 4. Addition of nutrients which may or may not originally be present |
QUESTION ID:124
| Column I | Column II |
| P. Milk | 1. Colloidal |
| Q. Butter | 2. Solution |
| R. Lactose | 3. Water in oil emulsion |
| S. Casein | 4. Oil in water emulsion |
QUESTION ID:125
 TLS Online
TLS Online