Performance Meter
0%
QUESTION ID:1
QUESTION ID:2
QUESTION ID:3
QUESTION ID:4
QUESTION ID:5
QUESTION ID:6
QUESTION ID:7
QUESTION ID:8
QUESTION ID:9
QUESTION ID:10
QUESTION ID:11
QUESTION ID:12
QUESTION ID:13
QUESTION ID:14
QUESTION ID:15
QUESTION ID:16
QUESTION ID:17
QUESTION ID:18
QUESTION ID:19
The major product M in the reaction
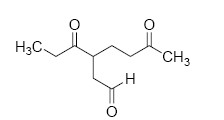
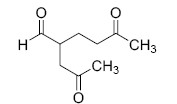
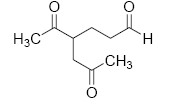
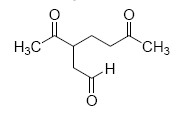
QUESTION ID:20
The two major products of the reaction
are
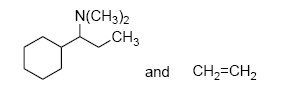
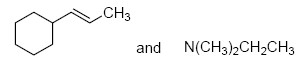
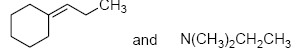
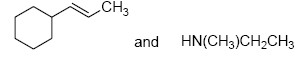
QUESTION ID:21
QUESTION ID:22
QUESTION ID:23
QUESTION ID:24
QUESTION ID:25
QUESTION ID:26
QUESTION ID:27
QUESTION ID:28
QUESTION ID:29
Which one of the following profiles represent the phenomenon of cooperativity?
QUESTION ID:30
QUESTION ID:31
QUESTION ID:32
QUESTION ID:33
QUESTION ID:34
QUESTION ID:35
QUESTION ID:36
QUESTION ID:37
| Group I | Group II |
| P. Increasing concentration of sodium chloride | i. Phenyl-Sepharose |
| Q. Increasing concentration of histidine | ii. Chromatofocusing |
| R. Decreasing concentration of ammonium sulphate | iii. DEAE-Sephacryl |
| S. Decreasing concentration of H+ | iv. Ni-NTA |
QUESTION ID:38
QUESTION ID:39
QUESTION ID:40
QUESTION ID:41
QUESTION ID:42
QUESTION ID:43
QUESTION ID:44
QUESTION ID:45
QUESTION ID:46
QUESTION ID:47
QUESTION ID:48
QUESTION ID:49
QUESTION ID:50
QUESTION ID:51
QUESTION ID:52
QUESTION ID:53
QUESTION ID:54
QUESTION ID:55
QUESTION ID:56
QUESTION ID:57
QUESTION ID:58
QUESTION ID:59
| Fruit character | Family | Plant species |
| P. Syconus | 1. Moraceae | i. Canavalia ensiformis |
| Q. Capsule, opening by apical pores or valves | 2. Fabaceae | ii. Artabotrys odoratissimus |
| R. Legume | 3. Papaveraceae | iii. Ficus religiosa |
| S. An etaerio of drupe | 4. Annonaceae | iv. Papaver somniferum |
| v. Pistacia vera | ||
| vi. Citrus aurantium |
QUESTION ID:60
| Disease | Affected plant | Causal organism |
| P. Black rot | 1. Corn | i. Fusarium oxysporum f.sp. cube |
| Q. Loose smut | 2. Banana | ii. Acidovorax avenae subsp. citrulli |
| R. Panama wilt | 3. Watermelon | iii. Botryosphaeria obtusa |
| S. Bacterial fruit blotch | 4. Apple | iv. Ustilago maydis |
| v. Plasmopara viticola | ||
| vi. Venturia inaequalis |
QUESTION ID:61
| Group-I | Group-II |
| P. Photorespiration | 1. Glutamate → 2-Oxoglutarate |
| Q. Respiration | 2. Acetyl-CoA → Malonyl-CoA |
| R. Amino acid degradation | 3. 2-Oxoglutarate → Succinyl-CoA |
| S. Fatty acid synthesis | 4. Glycine → Serine |
QUESTION ID:62
| Alkaloid | Use | Source species |
| P. Codeine | 1. Stimulant | i. Hyoscyamus niger |
| Q. Caffeine | 2. Analgesic | ii. Catharanthus roseus |
| R. Scopolamine | 3. Antineoplastic | iii. Cola nitida |
| S. Vinblastine | 4. Anticholinergic | iv. Papaver somniferum |
| v. Coptis japonica | ||
| vi. Senecio jacobaea |
QUESTION ID:63
QUESTION ID:64
QUESTION ID:65
QUESTION ID:66
QUESTION ID:67
QUESTION ID:68
QUESTION ID:69
QUESTION ID:70
QUESTION ID:71
QUESTION ID:72
QUESTION ID:73
QUESTION ID:74
QUESTION ID:75
QUESTION ID:76
QUESTION ID:77
| Group I | Group II |
| (i) Fluoroquinolones | (p) beta lactam antimicrobial |
| (ii) Amphotericin B | (q) inhibition of protein synthesis |
| (iii) Tetracycline | (r) inhibition of nucleic acid synthesis |
| (iv) Amoxicillin | (s) antifungal agent |
QUESTION ID:78
| Microorganism | Mode of Transmission |
| (i) Bordetella pertussis | (p) Vector-borne |
| (ii) Dengue virus | (q) Blood-borne |
| (iii) Entamoeba histolytica | (r) Droplet infection |
| (iv) Hepatitis B virus | (s) Contaminated food |
QUESTION ID:79
| Precursor/Intermediates | Metabolic pathway |
| (i) Inosine monophosphate | (p) L-methionine biosynthesis |
| (ii) Ornithine | (q) L-tryptophan biosynthesis |
| (iii) Chorismate | (r) Purine biosynthesis |
| (iv) Homocysteine | (s) L-arginine biosynthesis |
QUESTION ID:80
| Scientists | Area of major contribution |
| (i) Antonie van Leeuwenhoek | (p) Taxonomy |
| (ii) Carl Linnaeus | (q) Antimicrobial agents |
| (iii) Sir Alexander Fleming | (r) Vaccination |
| (iv) Louis Pasteur | (s) Microscopy |
QUESTION ID:81
QUESTION ID:82
QUESTION ID:83
QUESTION ID:84
QUESTION ID:85
QUESTION ID:86
QUESTION ID:87
QUESTION ID:88
QUESTION ID:89
QUESTION ID:90
QUESTION ID:91
QUESTION ID:92
QUESTION ID:93
QUESTION ID:94
QUESTION ID:95
QUESTION ID:96
| Column I | Column II |
| I) African tick bite fever | i) Trypanosoma gambiense |
| II) Yellow fever | ii) Zika virus |
| III) Microcephaly | iii) Rickettsia sp. |
| IV) Sleeping sickness | iv) Flavivirus |
QUESTION ID:97
QUESTION ID:98
QUESTION ID:99
QUESTION ID:100
| Column I (Enzyme/Protein) | Column II (Location/Association) |
| I) Galactosyl transferase | (i) Vesicles |
| II) Cytochrome oxidase | (ii) Cytosol |
| III) Clathrin | (iii) Golgi complex |
| IV) Tubulin | (iv) Mitochondria |
QUESTION ID:101
QUESTION ID:102
| Column I | Column II |
| I) Tapeworm | (i) Bioluminescence |
| II) Jellyfish | (ii) Viviparous |
| III) Trichinella | (iii) Lateral heart |
| IV) Earthworm | (iv) Microvilli on the body surface |
QUESTION ID:103
QUESTION ID:104
QUESTION ID:105
QUESTION ID:106
QUESTION ID:107
QUESTION ID:108
QUESTION ID:109
QUESTION ID:110
QUESTION ID:111
QUESTION ID:112
QUESTION ID:113
QUESTION ID:114
QUESTION ID:115
QUESTION ID:116
| Group I | Group II |
| P. Ginger | 1. Lutein |
| Q. Green tea | 2. Gingerol |
| R. Spinach | 3. Curcumin |
| S. Turmeric | 4. Epigallocatechin gallate |
QUESTION ID:117
| Group I | Group II |
| P. Extraction | 1. Phospholipids |
| Q. Degumming | 2. Free fatty acids |
| R. Neutralization | 3. Pigments |
| S. Bleaching | 4. Crude oil |
QUESTION ID:118
| Group I (Spoilage Type) | Group II (Causative Microorganism) |
| P. Green rot of eggs | 1. Micrococcus spp. |
| Q. Putrid swell in canned fish | 2. Serratia marcescens |
| R. Red bread | 3. Pseudomonas fluorescens |
| S. Yellow discoloration of meat | 4. Clostridium sporogenes |
QUESTION ID:119
| Group I | Group II |
| P. Sake | 1. Milk |
| Q. Chhurpi | 2. Cabbage |
| R. Natto | 3. Rice |
| S. Sauerkraut | 4. Soybean |
QUESTION ID:120
| Group I | Group II |
| P. Cleaning | 1. Quality separation |
| Q. Grading | 2. Clarification |
| R. Size reduction | 3. Screening |
| S. Filtration | 4. Comminution |
QUESTION ID:121
QUESTION ID:122
QUESTION ID:123
QUESTION ID:124
QUESTION ID:125
 TLS Online
TLS Online