Performance Meter
0%
QUESTION ID:1
QUESTION ID:2
QUESTION ID:3
QUESTION ID:4
QUESTION ID:5
QUESTION ID:6
QUESTION ID:7
QUESTION ID:8
QUESTION ID:9
QUESTION ID:10
QUESTION ID:11
QUESTION ID:12
QUESTION ID:13
QUESTION ID:14
QUESTION ID:15
The total number of stereoisomers possible for the following structure is __
QUESTION ID:16
For the reaction mechanism,
the rate law is
QUESTION ID:17
QUESTION ID:18
The reactants P and Q in the following reaction are
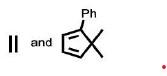
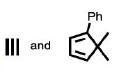
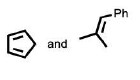
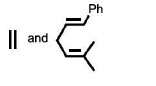
QUESTION ID:19
The major product formed in the following reaction is
QUESTION ID:20
The most stable coordination complexes P and Q formed in the following reaction are
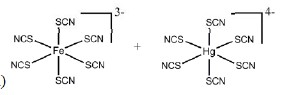
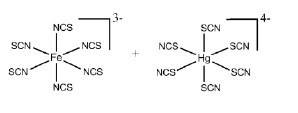
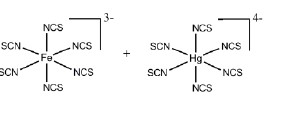
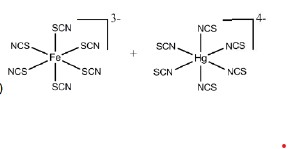
QUESTION ID:21
QUESTION ID:22
QUESTION ID:23
QUESTION ID:24
QUESTION ID:25
QUESTION ID:26
QUESTION ID:27
QUESTION ID:28
QUESTION ID:29
The dipeptide with least rotational barrier in the peptide bond is
QUESTION ID:30
QUESTION ID:31
QUESTION ID:32
QUESTION ID:33
QUESTION ID:34
QUESTION ID:35
QUESTION ID:36
QUESTION ID:37
QUESTION ID:38
QUESTION ID:39
QUESTION ID:40
QUESTION ID:41
QUESTION ID:42
QUESTION ID:43
QUESTION ID:44
QUESTION ID:45
QUESTION ID:46
QUESTION ID:47
QUESTION ID:48
QUESTION ID:49
QUESTION ID:50
QUESTION ID:51
QUESTION ID:52
QUESTION ID:53
QUESTION ID:54
QUESTION ID:55
QUESTION ID:56
QUESTION ID:57
QUESTION ID:58
QUESTION ID:59
QUESTION ID:60
QUESTION ID:61
QUESTION ID:62
QUESTION ID:63
QUESTION ID:64
QUESTION ID:65
QUESTION ID:66
QUESTION ID:67
QUESTION ID:68
QUESTION ID:69
Microbes make up about _____ of erath's living material by weight
QUESTION ID:70
QUESTION ID:71
QUESTION ID:72
QUESTION ID:73
QUESTION ID:74
QUESTION ID:75
QUESTION ID:76
QUESTION ID:77
QUESTION ID:78
QUESTION ID:79
QUESTION ID:80
QUESTION ID:81
QUESTION ID:82
QUESTION ID:83
QUESTION ID:84
QUESTION ID:85
QUESTION ID:86
QUESTION ID:87
QUESTION ID:88
QUESTION ID:89
QUESTION ID:90
QUESTION ID:91
QUESTION ID:92
QUESTION ID:93
QUESTION ID:94
QUESTION ID:95
QUESTION ID:96
QUESTION ID:97
QUESTION ID:98
QUESTION ID:99
QUESTION ID:100
QUESTION ID:101
| Column I (Hormones) | Column II (Physiological Effects) |
| a) Melatonin | 1) Inhibition of secretion of growth hormone |
| b) Oxytocin | 2) Synthesis of milk in mammary gland |
| c) Cholecystokinin | 3) Secretion of milk and uterine contraction |
| d) Prolactin | 4) Secretion of enzymes from pancreatic acinar cells |
| e) Somatostatin | 5) Regulation of circadian rhythms |
QUESTION ID:102
QUESTION ID:103
QUESTION ID:104
QUESTION ID:105
QUESTION ID:106
QUESTION ID:107
QUESTION ID:108
QUESTION ID:109
QUESTION ID:110
QUESTION ID:111
QUESTION ID:112
QUESTION ID:113
QUESTION ID:114
QUESTION ID:115
QUESTION ID:116
QUESTION ID:117
QUESTION ID:118
QUESTION ID:119
QUESTION ID:120
QUESTION ID:121
QUESTION ID:122
QUESTION ID:123
QUESTION ID:124
QUESTION ID:125
 TLS Online
TLS Online