Performance Meter
0%
QUESTION ID:1
Rajiv Gandhi Khel Ratna Award was conferred Mary Kom, a six-time world champion in boxing, recently in a ceremony the Rashtrapati Bhawan (the President's official residence) in New Delhi.
QUESTION ID:2
Despite a string of poor performances, the chances of K. L. Rahul's selection in the team are
QUESTION ID:3
QUESTION ID:4
QUESTION ID:5
QUESTION ID:6
QUESTION ID:7
QUESTION ID:8
The distance between Delhi and Agra is 233 km. A car P started travelling from Delhi to Agra and another car Q started from Agra to Delhi along the same road 1 hour after the car P started. The two cars crossed each other 75 minutes after the car Q started. Both cars were travelling at constant speed. The speed of car P was 10 km/hr more than the speed of car O. How many kilometers the car Q had travelled when the cars crossed each other?
QUESTION ID:9
QUESTION ID:10
QUESTION ID:11
An aqueous solution contains a mixture of 10-8 M NaCI and 10-8 M HCI. Choose the correct statement about this solution.
QUESTION ID:12
QUESTION ID:13
In naphthalene, the value of the integer "n" according to Huckel's rule of aromaticity is
QUESTION ID:14
The azimuthal quantum number (I) of an electron in the cl,= orbital of a copper atom (atomic number: 29) is
QUESTION ID:15
QUESTION ID:16
The correct order of the first ionization energies of He, B, N and 0 in their corresponding ground state is
QUESTION ID:17
Based on the molecular orbital theory, which one of the following statements with respect to N2, N2+, O2 and O2+ is correct?
QUESTION ID:18
Which one of the following statements is incorrect about the diborane molecule?
QUESTION ID:19
QUESTION ID:20
QUESTION ID:21
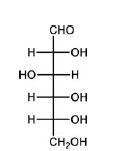
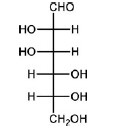
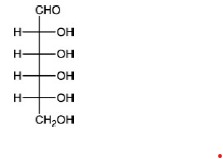
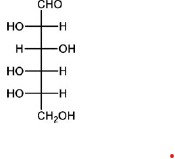
QUESTION ID:22
A molecule in solution crystallizes into two different crystal forms with rate constants of 0.02 s-1 and 0.13s-1 If the crystallization is assumed to be under kinetic control, then the half-life (in seconds, rounded off to one decimal place) of the molecule is
QUESTION ID:23
The standard potential 010 for a cell reaction given below is +0.7 V. The standard reaction free energy (11,G9) for this cell is kJ mol-1 (correct up to two decimal places). (Given: Faraday constant,
QUESTION ID:24
The activation energy (Ea) estimated for a reaction from the Arrhenius equation is 21KJ mol-1. If the frequency factor is assumed to be independent of temperature, then the ratio of the rate constants determined at 298 K and 260 K is (rounded off to two decimal places). (Given: Gas constant, R = 8.315 J K.-1 mo1-1)
QUESTION ID:25
QUESTION ID:26
Which one of the following hormones initiates a signaling cascade by directly binding to an intra-cellular receptor?
QUESTION ID:27
Which one of the following bonds is NOT present in ATP?
QUESTION ID:28
The reaction involved in the direct conversion of L-phenylalanine to L-tyrosine is
QUESTION ID:29
The human major histocompatibility complex (MHC) is
QUESTION ID:30
Har Gobind Khorana and Marshall Nirenberg elucidated the genetic code by using a cell-free protein synthesizing system. It was found that poly(U) and poly(C) result in the synthesis of poly(L-Phe) and poly(L-Pro), respectively. Based on these observations, which one of the following conclusions is correct?
QUESTION ID:31
QUESTION ID:32
QUESTION ID:33
Choose the correct order of molecules according to their ability to diffuse across a lipid bilayer.
QUESTION ID:34
When one glucose unit from glycogen gets converted to lactate in the muscle, the net number of ATP molecules produced is
QUESTION ID:35
Considering that the three pKas of histidine are pK1=1.8, pK2=9.2 and pKR=6.0, its isoelectric point will be (rounded off to one decimal place).
QUESTION ID:36
One mole of a native protein upon N-terminal analysis yielded one mole each of Asp and Val. Therefore, the protein in its native state exists as a
QUESTION ID:37
The prosthetic groups/cofactors involved in both le- and 2e- transfer in the mitochondrial electron transport chain are
QUESTION ID:38
QUESTION ID:39
QUESTION ID:40
Polypeptides are either biosynthesized on the ribosomes using an mRNA template or chemically synthesized by the Merrifield's solid phase method. The correct directions of peptide synthesis are
QUESTION ID:41
A solution absorbs 20% of the incident light in a cuvette of path length 1.0 cm. The amount of light transmitted by the same solution in a cuvette of 3.0 cm path length is % (rounded off to one decimal place).
QUESTION ID:42
The second pKa of phosphoric acid is 6.8. The ratio of Na2HPO4 to NaH2PO4 required to obtain a buffer of pH 7.0 is (rounded off to two decimal places).
QUESTION ID:43
A PCR in a 100 Micro L reaction volume, containing two primers at a concentration of 0.2 Micro M each, is set up to amplify a 250 base pair DNA fragment. Consider the average molecular weight of one base pair as 660 Da. If the primers are fully consumed by the end of the reaction, the amount of the final PCR product formed is Micro g (rounded off to one decimal place).
QUESTION ID:44
An enzyme obeying Michaelis-Menten kinetics shows a reaction velocity (v) of 10 micro mol/min when the substrate concentration [S] equals its Km. The maximal velocity Vmax for this enzyme is Micro mol/min (correct to integer number). (KM is Michaelis-Menten constant)
QUESTION ID:45
QUESTION ID:46
Indefinite stamen is a characteristic feature of which of the following plant families?
QUESTION ID:47
In natural condition, which of the following plants DOES NOT exhibit anomalous secondary growth?
QUESTION ID:48
In a typical angiosperm under natural condition, primary meristems are usually established during
QUESTION ID:49
2-Methoxy-3, 6-dichlorobenzoic acid belongs to which class of plant growth regulators?
QUESTION ID:50
In a typical green plant, the first stable product of Calvin cycle is
QUESTION ID:51
Among the following, which best describes an organism that lives at the expense of other organisms, harmful but usually not killing?
QUESTION ID:52
The oleo-gum resin asafoetida (hing) is obtained from the cut surface or
QUESTION ID:53
Bakanae' disease or 'foolish seedling' disease is caused by
QUESTION ID:54
QUESTION ID:55
An mRNA of a nuclear encoded plant gene, DSH2O has an ORF of 1353 nucleotides. Provided that average molecular weight of amino acid is 110 Dalton (Da), calculated molecular weight of DSH2O protein in kDa (round oil to I decimal place) is
QUESTION ID:56
QUESTION ID:57
QUESTION ID:58
QUESTION ID:59
QUESTION ID:60
QUESTION ID:61
QUESTION ID:62
QUESTION ID:63
QUESTION ID:64
QUESTION ID:65
QUESTION ID:66
The technique of microbial "pure culture" was pioneered by
QUESTION ID:67
The antibacterial trimethoprim is an inhibitor of
QUESTION ID:68
Choose the correct taxonomical hierarchy among the following:
QUESTION ID:69
Shifting a Saccharomyces cerevisiae culture from fermentative to aerobic respiratory mode will
QUESTION ID:70
Which one of the following diseases is treated by a neuraminidase inhibitor?
QUESTION ID:71
Which one of the following does NOT provide three-dimensional images?
QUESTION ID:72
Which one of the following will increase the resolution of a light microscope?
QUESTION ID:73
Which one of the following conditions favors maximum expression of lac operon genes in E. coli?
QUESTION ID:74
QUESTION ID:75
QUESTION ID:76
Digestion of an immunoglobulin G (lgG) molecule with pepsin will NOT
QUESTION ID:77
QUESTION ID:78
QUESTION ID:79
Which one of the following statements about control of microbial growth is NOT correct?
QUESTION ID:80
An example of a differential and selective medium in which colonies of Gram-negative bacteria produce large amounts of acidic products and appear green with a metallic sheen is
QUESTION ID:81
QUESTION ID:82
A bacterial culture containing 3 x 105 live cells was exposed to a newly developed sterilizing agent. After 30 minutes of exposure, 3 live cells remained in culture. The decimal reduction time (in minutes) for the new agent is
QUESTION ID:83
QUESTION ID:84
QUESTION ID:85
QUESTION ID:86
Which ONE of following leucocytes is phagocytic and has clear cytoplasm?
QUESTION ID:87
QUESTION ID:88
Which ONE of the following is NOT a site for in situ conservation?
QUESTION ID:89
Which ONE of the following is the precursor molecule for corticosteroids?
QUESTION ID:90
Transitional epithelia is found in which ONE of the following organs?
QUESTION ID:91
Visual signal transduction cascade is activated by rhodopsin and involves degradation rather than synthesis of which ONE of the following second messenger molecules?
QUESTION ID:92
The genomes of both human and Drosophila code for an amylase that acts on the same substrate. However, the sequence of nucleotides in the genes encoding the two is dissimilar. This is an example of which ONE of the following types of evolution?
QUESTION ID:93
"Round dance" is performed by forager bees to indicate the distance between a food source and their colony. Which ONE of the following best represents this distance?
QUESTION ID:94
Which ONE of the following phyla have choanocytes?
QUESTION ID:95
QUESTION ID:96
QUESTION ID:97
Match the following subcellular organelles in Column I with associated functions in Column II
QUESTION ID:98
QUESTION ID:99
QUESTION ID:100
QUESTION ID:101
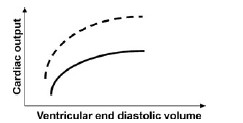
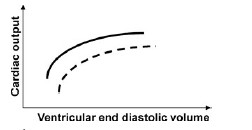
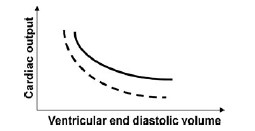
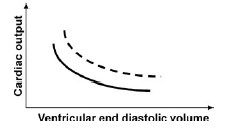
QUESTION ID:102
QUESTION ID:103
QUESTION ID:104
QUESTION ID:105
QUESTION ID:106
The enzyme majorly involved in postmortem degradation of muscle proteins is
QUESTION ID:107
Which of the following is the correct pair of essential fatty acids?
QUESTION ID:108
Nisin A is produced by
QUESTION ID:109
Which of the following bacteria will stain purple color after Gram staining?
QUESTION ID:110
The enzyme system used for removal of glucose from egg white prior to its drying consists of
QUESTION ID:111
The INCORRECT pair of food borne illness and its causative microorganism is
QUESTION ID:112
Which of the following is commonly used as a preservative in the tomato sauce?
QUESTION ID:113
A fluid with flow behaviour index less than one (n < I) is
QUESTION ID:114
The velocity of 2.2 gm diameter fat particles inside a centrifuge, running at 6000 rpm and 20 °C, is 0.25 mm s-1. The velocity of 1.5 .tm diameter fat particles inside the same centrifuge running at 7500 rpm and same temperature (round off to 2 decimal places) will be mm s-1
QUESTION ID:115
The initial population of a bacterial strain increases from 1x104 cells per mL to 1x106 cells per mL in 120 minutes. The generation time for this strain (round off to 2 decimal places) is minutes.
QUESTION ID:116
QUESTION ID:117
QUESTION ID:118
QUESTION ID:119
QUESTION ID:120
QUESTION ID:121
QUESTION ID:122
QUESTION ID:123
QUESTION ID:124
QUESTION ID:125
An orange juice flowing at 0.80 kg s' enters a counter current double pipe heat exchanger at 20 °C and leaves at 72 °C. Inlet and outlet temperatures of the hot water used as heating medium in the exchanger are 81 °C and 74 °C, respectively. The specific heat of the orange juice is 3.74 kJ kg-1 K-1and overall heat transfer coefficient is 492 W m 2 10. The heat transfer surface area (round off to 2 decimal places) will be m2.
 TLS Online
TLS Online