Performance Meter
0%
QUESTION ID:1
QUESTION ID:2
QUESTION ID:3
QUESTION ID:4
QUESTION ID:5
In the given diagram, ovals are marked at different heights (h) of a hill. Which one of the following options P, Q, R, and S depicts the top view of the hill?
QUESTION ID:6
QUESTION ID:7
QUESTION ID:8
QUESTION ID:9
Which one of the given figures P, Q, R and S represents the graph of the following function?
𝑓(𝑥)=| |𝑥+2|−|𝑥−1| |
QUESTION ID:10
An opaque cylinder (shown below) is suspended in the path of a parallel beam of light, such that its shadow is cast on a screen oriented perpendicular to the direction of the light beam. The cylinder can be reoriented in any direction within the light beam. Under these conditions, which one of the shadows P, Q, R, and S is NOT possible?
QUESTION ID:11
QUESTION ID:12
What is the major product formed in the given reaction?
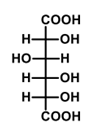
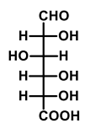
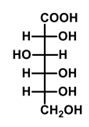
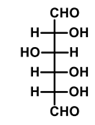
QUESTION ID:13
The CORRECT order of stability of the given metal oxides is
QUESTION ID:14
QUESTION ID:15
Suitable reagent(s) to bring about the conversion of P to Q in good yield is/are
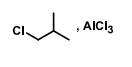
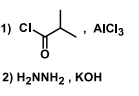
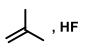
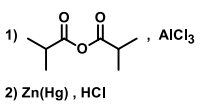
QUESTION ID:16
QUESTION ID:17
QUESTION ID:18
QUESTION ID:19
QUESTION ID:20
QUESTION ID:21
QUESTION ID:22
QUESTION ID:23
QUESTION ID:24
QUESTION ID:25
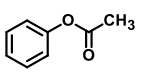
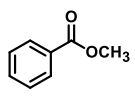
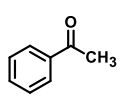
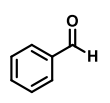
QUESTION ID:26
QUESTION ID:27
Eº = 1.10 V for the following cell reaction:
For this reaction, the equilibrium constant is 𝑦 × 1037 at 298 K. The value of 𝑦 is _____ (rounded off to two decimal places).
(Given: F = 96485 C molˉ1, R = 8.314 J Kˉ1molˉ1)
QUESTION ID:28
QUESTION ID:29
QUESTION ID:30
QUESTION ID:31
QUESTION ID:32
QUESTION ID:33
QUESTION ID:34
QUESTION ID:35
QUESTION ID:36
Which one of the following amino acids has more than two acid-base groups?
QUESTION ID:37

QUESTION ID:38
QUESTION ID:39
QUESTION ID:40
Match the cell types listed in Group I with associated processes listed in Group II.
QUESTION ID:41
QUESTION ID:42
QUESTION ID:43
QUESTION ID:44
QUESTION ID:45
QUESTION ID:46
QUESTION ID:47
Which one of the following statements on Casparian strips is correct?
QUESTION ID:48
QUESTION ID:49
QUESTION ID:50



QUESTION ID:51
QUESTION ID:52
QUESTION ID:53
QUESTION ID:54
QUESTION ID:55
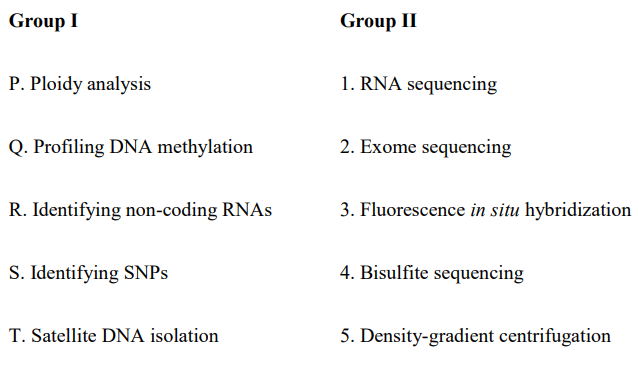
QUESTION ID:56
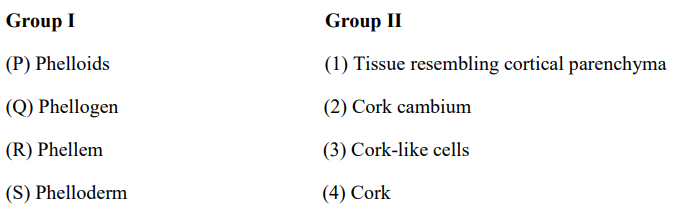
QUESTION ID:57

QUESTION ID:58
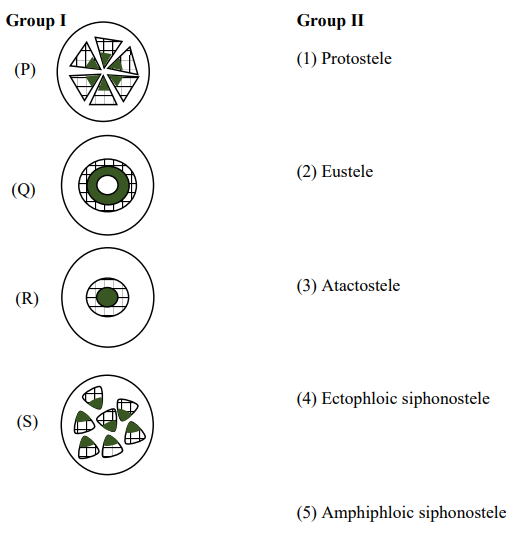
QUESTION ID:59
QUESTION ID:60
QUESTION ID:61
QUESTION ID:62
QUESTION ID:63
QUESTION ID:64
QUESTION ID:65
QUESTION ID:66
Which one of the following animals has “Book Lungs” as a respiratory organ?
QUESTION ID:67
Which one of the following describes the “innate behavior” of an animal?A behavior that is triggered due to the change in environment.
QUESTION ID:68
QUESTION ID:69
Which of the following animals show “Bottle cells” during the gastrulation stage of development?
QUESTION ID:70
organisms that obtain energy from inorganic compounds are known as
QUESTION ID:71
Which of the following is/are the causative agent(s) of Filariasis?
QUESTION ID:72
In a population of 1000 wild dogs in a grassland, 360 and 480 dogs had black body colour with genotypes BB and Bb, respectively. In the same population, remaining dogs were white in colour with a genotype of bb. Based on this data, the frequency of allele “b” in the population is ______ (round off to one decimal place).
QUESTION ID:73
A mature rat sperm cell has 2.5 μg of genomic DNA that is equivalent of a haploid genome. Compared to this sperm cell, the amount of genomic DNA (in μg) in a somatic cell, which is in the G2 phase of cell cycle, will be _____ (in integer).
QUESTION ID:74
In an experiment, excess amount of bicod mRNA (more than wild-type expression level) was injected into the posterior pole of a wild-type Drosophila embryo at pre-blastodermal stage. Out of the following options, which one represents the best expected phenotype in the resulted developing embryo?
QUESTION ID:75
Match the hormones/precursors listed in Column I with their chemical type in Column II and the tissue of origin listed in Column III
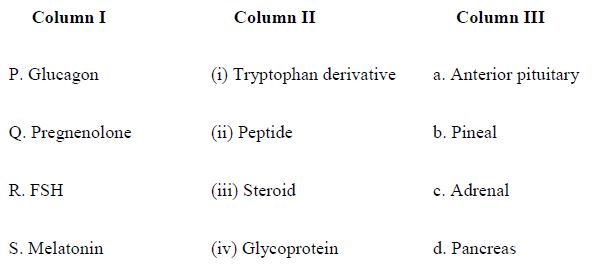
QUESTION ID:76
Match the syndromes listed in Column I with the cause/symptoms listed in Column II
QUESTION ID:77
Match the immunological statements in Column I with the appropriate descriptions from Column II
QUESTION ID:78
Match the standard/stated cofactors in Column I with their respective enzymes in Column II
QUESTION ID:79
The presence of excess glucose has been known to prevent the induction of lac operon as well as other operon controlling enzymes involved in carbohydrate metabolism in E. coli. Which of the following processes define(s) the phenomenon?
QUESTION ID:80
Which of the following techniques is/are used for determining the three-dimensional structure of proteins?
QUESTION ID:81
QUESTION ID:82
Which of the following statements is/are TRUE for Colchicine?
QUESTION ID:83
Wild-type Drosophila females having three linked genes (AABBCC) were crossed with triple recessive mutant (aabbcc) males. The F1 female progenies (AaBbCc) were back crossed with the triple negative mutant (aabbcc) males. The cross resulted in following number of progenies in F2 :
The order of genes as determined from the above data was found to be “ABC” (note that the order is equivalent to “CBA” and the order outside the markers are arbitrary).
The recombination map distance (in centi Morgan) between “A to C” is ______ (round off to one decimal place).
QUESTION ID:84
Monkey pox is caused by a
QUESTION ID:85
The length of a double helical DNA molecule is 13.6 km. If the DNA double helix weighs 1 ×10−18 g per 1000 nucleotide pairs and rise per base pair is 3.4 Å, then weight of the double helical DNA molecule (in nanogram) will be ______ (in integer).
QUESTION ID:86
Which one of the following converts sulfate to hydrogen sulfide?
QUESTION ID:87
Which one of the statements about bacterial flagella is correct?
QUESTION ID:88
Microbial plastics are made from
QUESTION ID:89
The correct sequence of metabolic intermediates in Krebs cycle is
QUESTION ID:90
Catabolite repression in bacteria is regulated by the concentration of
QUESTION ID:91
Phagocytosis was first described by
QUESTION ID:92
Which one of the following statements about batch culture of microbes is NOT correct?
QUESTION ID:93
Match the test in Group I with its application in Group II
Group I Group II
P. Oakley-Fulthorpe test 1. IgM detection
Q. Limulus amoebocyte lysate test 2. Determining antigen-antibody specificity
R. Weil-Felix reaction test 3. Endotoxin detection
S. Complement-fixation test 4. Rickettsial infection diagnosis
QUESTION ID:94
Which one of the following is NOT correct about antibiotic resistance mechanism in microbes?
QUESTION ID:95
A suspension of photosynthetic green algae was illuminated in the presence of 14CO2 for few seconds. The first metabolite in the Calvin cycle to be radiolabeled will be
QUESTION ID:96
QUESTION ID:97
Which one of the following conjugations will result in formation of merodiploids?
QUESTION ID:98
Which of the following genus is/are a spirochete(s)?
QUESTION ID:99
Which of the following is/are non-membrane bound inclusion bodies?
QUESTION ID:100
Which of the following antibiotics is/are isolated from Streptomyces spp.?
QUESTION ID:101
Which of the following statements about the primary and secondary adaptive immune responses to an antigen is/are correct?
QUESTION ID:102
The spontaneous, and induced mutations in bacteria can be distinguished by
QUESTION ID:103
During the exponential growth, it took 6 hours for the population of bacterial cells to increase from 2.5 × 106 to 5 × 108. The generation time of the bacterium, rounded off to the nearest integer, is ________ minutes.
QUESTION ID:104
Choose the correct group of fat soluble vitamins
QUESTION ID:105
QUESTION ID:106
QUESTION ID:107
The time required for stipulated destruction of a microbial population at a given temperature is
QUESTION ID:108
QUESTION ID:109
QUESTION ID:110
Orange juice is packaged aseptically and stored under ambient conditions. The degradation of vitamin C in the juice occurs during storage and it follows first order reaction kinetics. The degradation rate constant is 5.2×103 day¹. The half-life of vitamin C in days is (in integer).
QUESTION ID:111
QUESTION ID:112
Some of the industrial products are produced by fermentation processes. Identify the correct pair of product and fermentative microorganism.
QUESTION ID:113
QUESTION ID:114
QUESTION ID:115
QUESTION ID:116
Match the method value used for measuring lipid characteristics in Column I with the corresponding properties indicated by them, in Column II.
Column I Column II
P. Thiobarbituric acid test 1. Induction time
Q. Rancimat method 2. Degree of unsaturation
R. Peroxide value 3. Carbonyl content
S, Iodine value 4. Hydroperoxide content
QUESTION ID:117
QUESTION ID:118
QUESTION ID:119
QUESTION ID:120
A sample of glucose isomerase enzyme converts 15 umoles of substrate glucose into product fructose min¹ mL under standard assay conditions. The enzyme activity of the glucose isomerase in International Unit (IU) is (in integer)
QUESTION ID:121
If D10 for Salmonella in egg yolk is 0.75 kGy, calculate the radiation dose in kGy (rounded off to 2 decimal places) required for reducing the Salmonella count in egg yolk by 8 log cycles.
QUESTION ID:122
The average moisture binding energy of a textured protein product (TPP) at 8% moisture content (dry basis) is 3200 cal.mol'. If the water activity of the TPP at the above moisture content is 0.30 at 30 °C, the water activity of the sample at 45 °C is (rounded off to 2 decimal places). The value of Gas constant R = 1.987 cal.mol'.K'.
 TLS Online
TLS Online