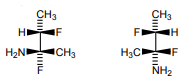TLS Online TPP Program
More Questions
TLS Online TPP Program
#Question id: 27063
#Bioorganic Chemistry and Metabolites
Determine R and S Configurations On A Fischer Projection given below
TLS Online TPP Program
#Question id: 10261
#Bioorganic Chemistry and Metabolites
What is the enthalpy change for the given reaction?
CH3CH3 + Cl2 → CH3CH2Cl + HCl
Given the enthalpies
| Bonds | Bond Enthalpy (kJ mol⁻¹) |
| C-H | 413 |
| Cl-Cl | 239 |
| C-C | 347 |
| C-Cl | 339 |
| H-Cl | 427 |
TLS Online TPP Program
#Question id: 27064
#Bioorganic Chemistry and Metabolites
These are three amino structure acid given below choose the correct option regarding the stereochemistry of these fig.
TLS Online TPP Program
#Question id: 27065
#Bioorganic Chemistry and Metabolites
Determine R and S Configurations On A Fischer Projection given below
TLS Online TPP Program
#Question id: 27066
#Bioorganic Chemistry and Metabolites
Determine R and S Configurations On A Fischer Projection given below
TLS Online TPP Program
#Question id: 27068
#Bioorganic Chemistry and Metabolites
Isoleucine and Threonine have two chiral carbons so exists in multiple RS configuration, choose the correct option regarding the given fig.
1. a is D-threonine is (2S, 3S) threonine
2. b is L-threonine is (2R,3S) threonine
3. a is L-threonine is (2S, 3R) threonine
4. b is D-threonine is (2R,3S) threonine