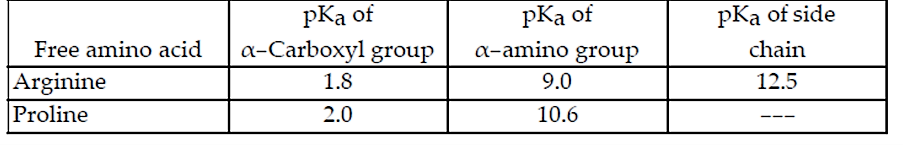
#Question id: 551
#Section 2: General Biology
Enzymes differ from other catalysts in that only enzymes:
#Question id: 552
#Section 2: General Biology
Which of the following is true of the binding energy derived from enzyme-substrate interactions?
#Question id: 553
#Section 2: General Biology
The concept of “induced fit” refers to the fact that:
#Question id: 554
#Section 2: General Biology
The Lineweaver-Burk plot is used to:
#Question id: 555
#Section 2: General Biology
The double-reciprocal transformation of the Michaelis-Menten equation, also called the Lineweaver-Burk plot, is given by To determine Km from a double-reciprocal plot, you would:
#Question id: 556
#Section 2: General Biology
Which of the following statement is correct to calculate the turnover number of an enzyme,
A) the enzyme concentration.
B) the initial velocity of the catalyzed reaction at [S] >> Km.
C) the initial velocity of the catalyzed reaction at low [S].
D) the Km for the substrate.