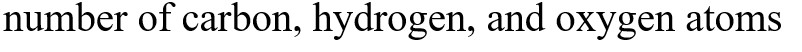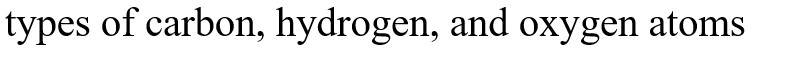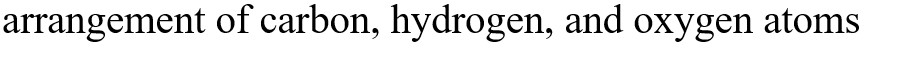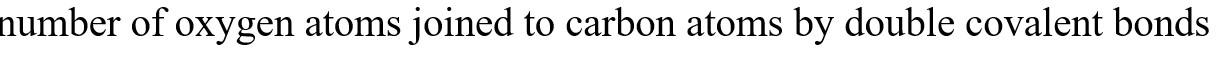#Question id: 36
#Bioorganic Chemistry and Metabolites
Which of the following is/are the S-enantiomer of alanine?
#Question id: 39
#Bioorganic Chemistry and Metabolites
What functional groups are present in this molecule?
#Question id: 38
#Bioorganic Chemistry and Metabolites
Which of the following is capable of existing as a pair of enantiomers?
#Question id: 42
#Bioorganic Chemistry and Metabolites
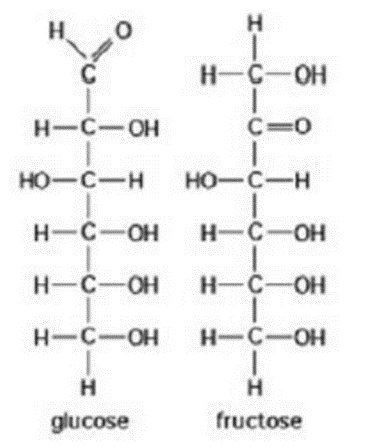
What is the relation between the given compound?
#Question id: 48
#Bioorganic Chemistry and Metabolites
Which of the following statements regarding enantiomers true?
#Question id: 49
#Bioorganic Chemistry and Metabolites
The equilibrium constant, Keq, for the following reaction is 2 × 105 M: If the measured cellular concentrations are [ATP] = 5 mM, [ADP] = 0.5 mM, and [Pi] = 5 mM, on the basis of the above information calculate the Keq in living cells?