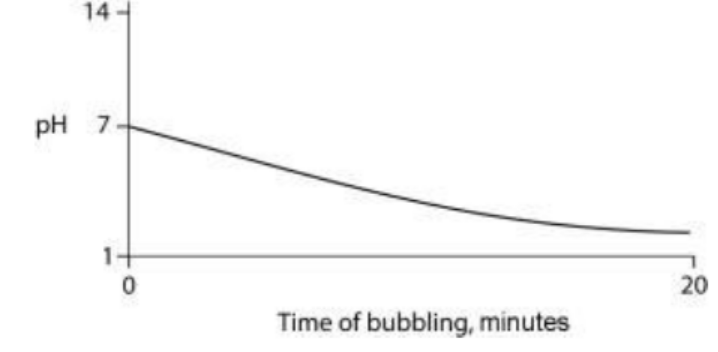
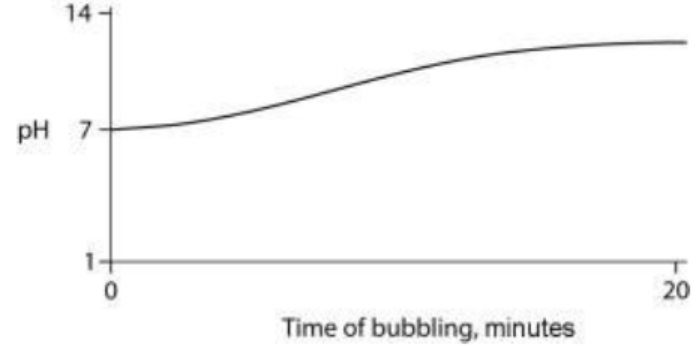
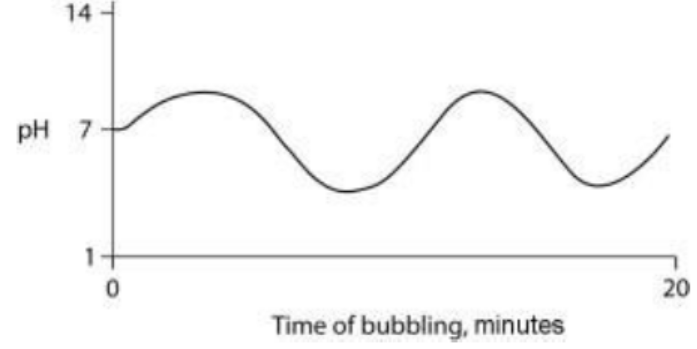
TLS Online TPP Program
More Questions
TLS Online TPP Program
#Question id: 565
#Section 2: General Biology
A transition-state analog:
TLS Online TPP Program
#Question id: 566
#Section 2: General Biology
The role of the metal ion (Mg2+) in catalysis by enolase is to
TLS Online TPP Program
#Question id: 567
#Section 2: General Biology
Penicillin and related drugs inhibit the enzyme; this enzyme is produced by
TLS Online TPP Program
#Question id: 568
#Section 2: General Biology
Which of the following statements about allosteric control of enzymatic activity is false?
TLS Online TPP Program
#Question id: 569
#Section 2: General Biology
A small molecule that decreases the activity of an enzyme by binding to a site other than the catalytic site is termed a(n):
TLS Online TPP Program
#Question id: 570
#Section 2: General Biology
Which of the following is true for allosteric enzymes