TLS Online TPP Program
More Questions
TLS Online TPP Program
#Question id: 19596
#SCPH05 I Biotechnology
For an ideal gas, CV and CP are related as :
TLS Online TPP Program
#Question id: 19597
#SCPH05 I Biotechnology
The least random state of the water system is:
TLS Online TPP Program
#Question id: 19598
#SCPH05 I Biotechnology
Considering entropy(S) thermodynamic parameters the criteria for the spontaneity of any process is:
TLS Online TPP Program
#Question id: 19599
#SCPH05 I Biotechnology
The enthalpy change in a reaction does not depend upon
TLS Online TPP Program
#Question id: 19600
#SCPH05 I Biotechnology
The correct relationship between free energy change in a reaction and the corresponding equilibrium constant KC is
TLS Online TPP Program
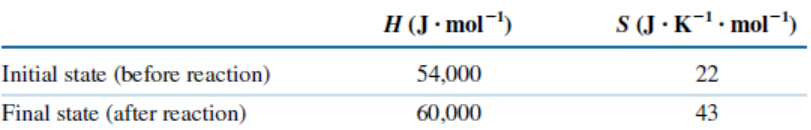
#Question id: 19601
#SCPH05 I Biotechnology
What is the entropy change (in JK-1 mol-1) when 1 mole of ice is converted into water at 0℃? (The enthalpy change for the conversion of ice to liquid water is 6.0 kJ mol-1 at 0℃)